TIDES TRIAL
Explore the pivotal Phase 3 TIDES study on vaccine efficacy
and safety profile of TAK-003.11,21
and safety profile of TAK-003.11,21
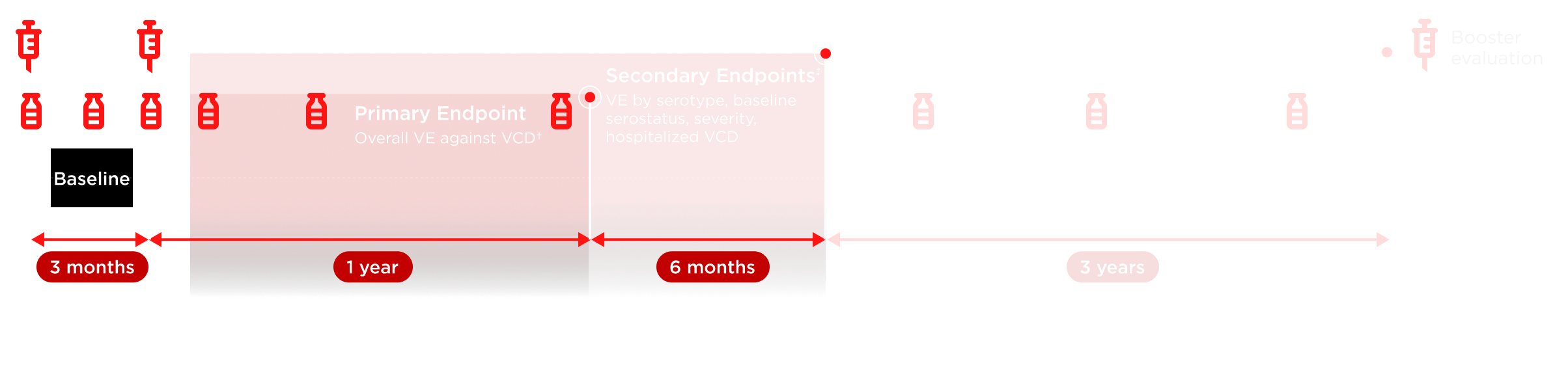
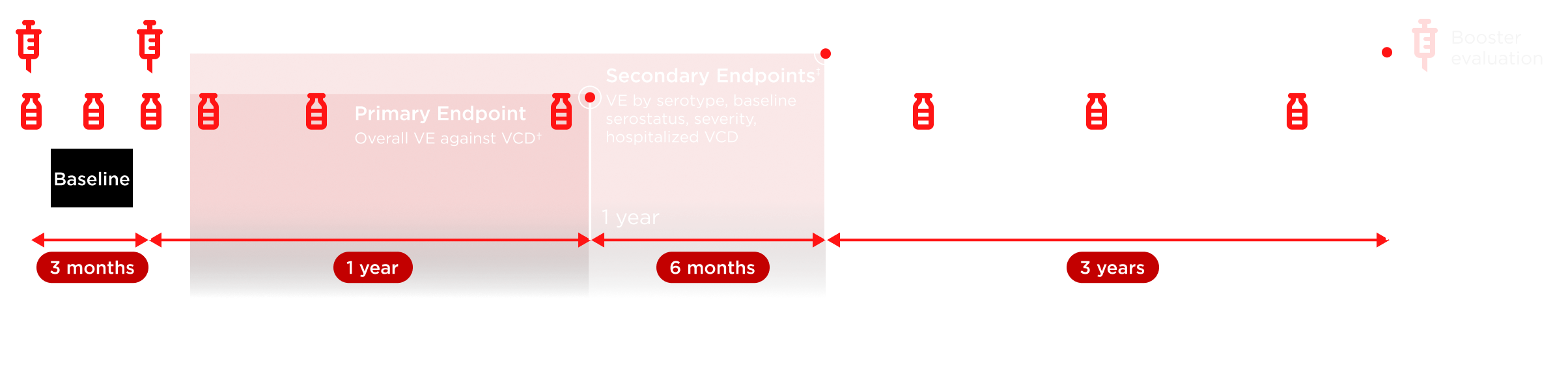
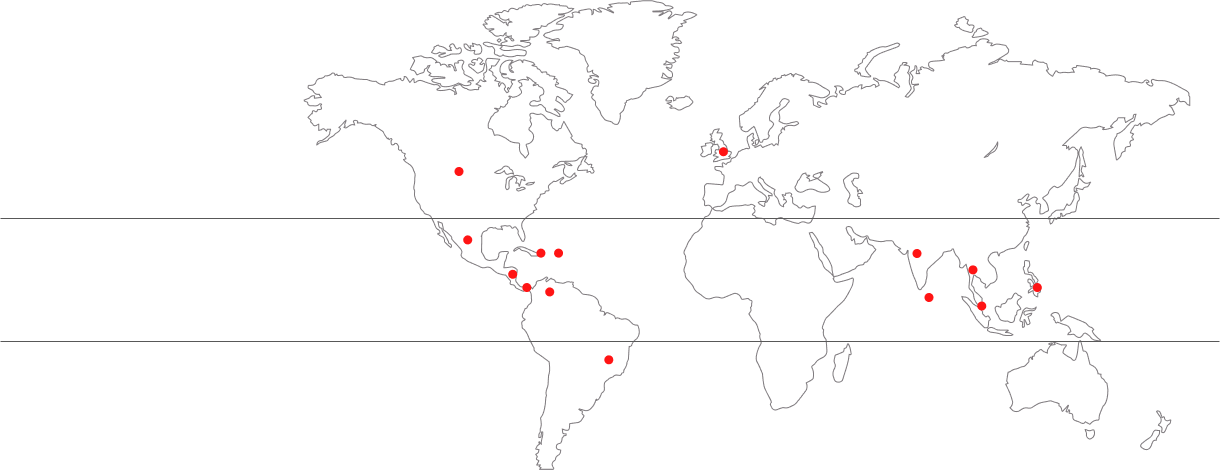
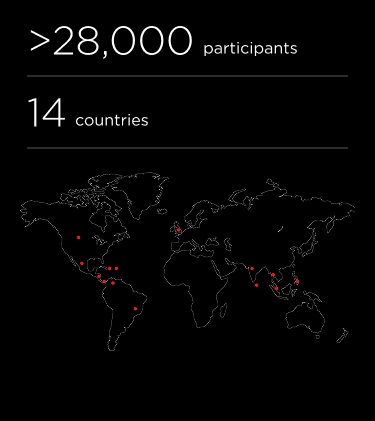
TIDES, Tetravalent Immunization Against Dengue Efficacy Study; VCD, virologically confirmed dengue; VE, vaccine efficacy.
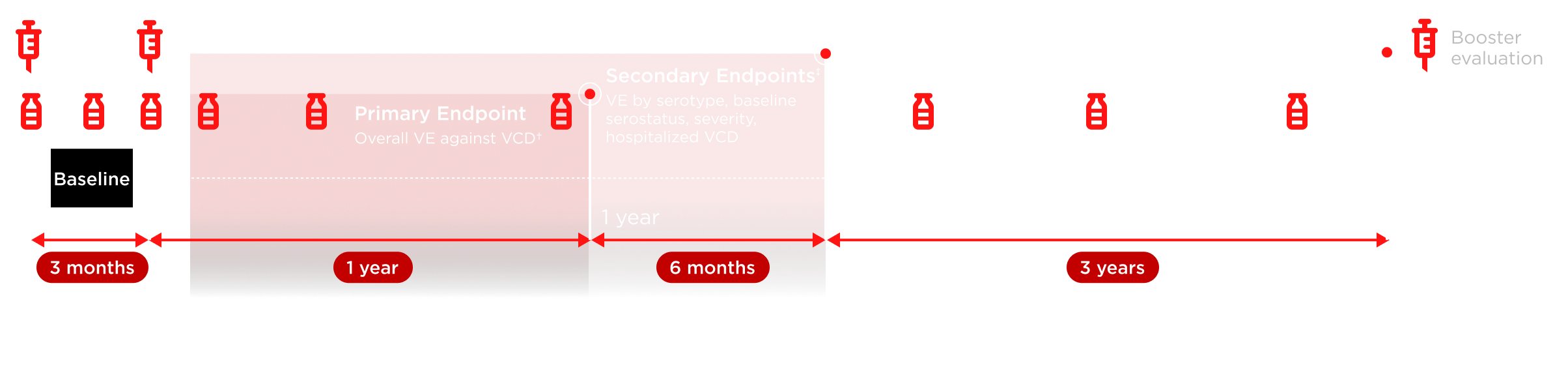
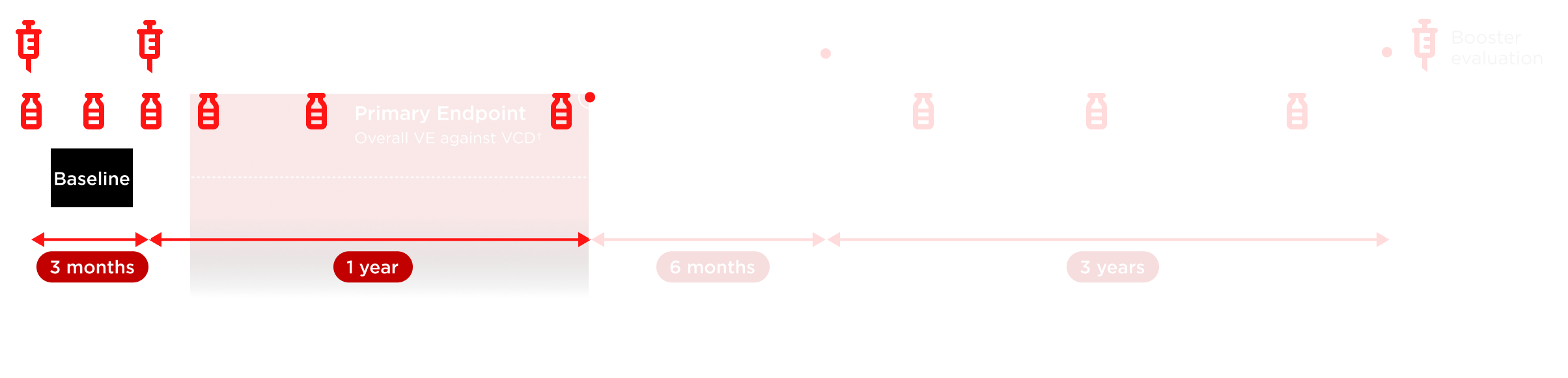
*Microneutralization testing of 3,765 participants (per protocol) was carried out pre-vaccination on Day 1, post-first dose at Month 1, pre-vaccination at Month 3, post-second dose at Months 4, 9, and 15, and then annually (up to 3 years). †VE defined as 1 – (λV/λC), where λV and λC denote the hazard ratios for developing VCD fever for the TAK-003 and placebo arms, respectively. Number of participants evaluated each year may vary. Repeat episodes of VCD excluded from efficacy analysis. ‡Endpoints were not exclusively secondary at 18 months.
TIDES, Tetravalent Immunization Against Dengue Efficacy Study; VCD, virologically confirmed dengue; VE, vaccine efficacy.
*Microneutralization testing of 3,765 participants (per protocol) was carried out pre-vaccination on Day 1, post-first dose at Month 1, pre-vaccination at Month 3, post-second dose at Months 4, 9, and 15, and then annually (up to 3 years). †VE defined as 1 – (λV/λC), where λV and λC denote the hazard ratios for developing VCD fever for the TAK-003 and placebo arms, respectively. Number of participants evaluated each year may vary. Repeat episodes of VCD excluded from efficacy analysis. ‡Endpoints were not exclusively secondary at 18 months.
TIDES PUBLICATIONS
SAFETY PUBLICATIONS
Review the safety profile of TAK-003, including the integrated safety analysis.
References
- NCT01224639.
- NCT01765426.
- NCT01542632.
- NCT01728792.
- NCT02193087.
- NCT01511250.
- NCT02302066.
- NCT02425098.
- NCT03746015.
- NCT02948829.
- NCT02747927.
- NCT03999996.
- NCT03423173.
- NCT03342898.
- NCT03771963.
- NCT04313244.
- NCT03525119.
- NCT03341637.
- NCT06060067.
- NCT01110551.
- Biswal S, et al. N Engl J Med. 2019;381(21):2009-2019.